Research
Cerebral Micro-circulation and Red Blood Cells
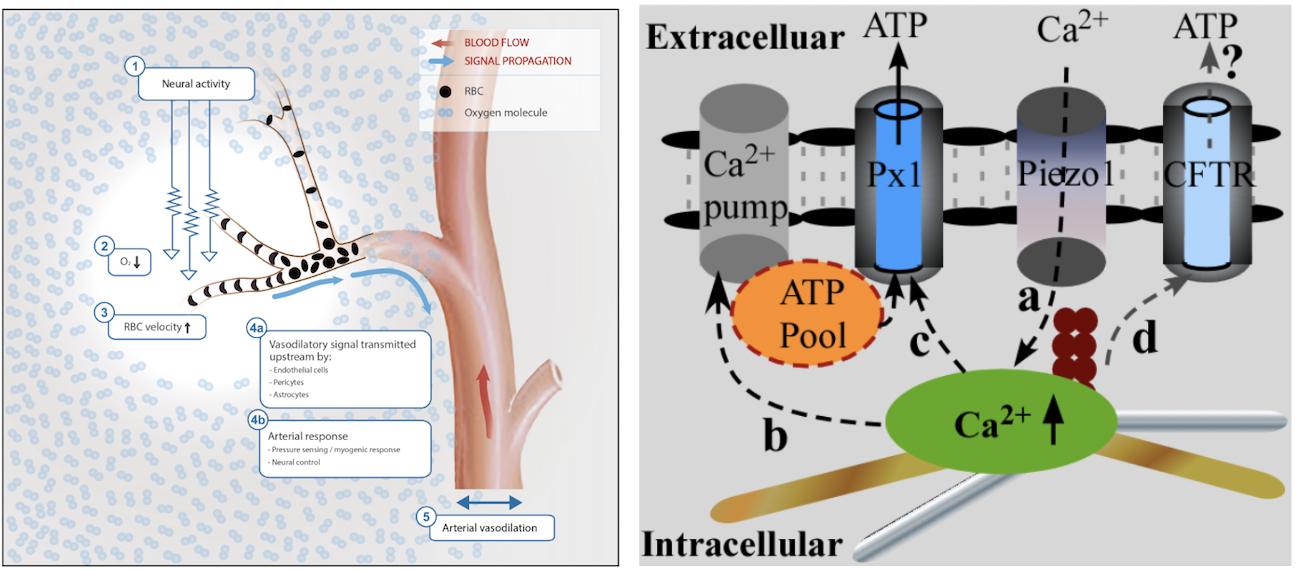
Neurovascular coupling or cerebral functional hyperemia forms the basis for functional brain imaging. Defects in functional hyperemia are believed to contribute to synaptic loss and cognitive decline in multiple neurodegenerative diseases, including Alzheimer disease. To date, significant efforts have been made to identify the mechanisms driving functional hyperemia but the results are debating. Indeed, majority of studies in this field focus on neurovascular unit (i.e., vascular walls, glial cells, and neurons) and the roles of red blood cells (RBCs) are significantly overlooked. Our lab has conducted the first formalized study and unveiled a previously unrealized regulatory role of RBCs in capillary hyperemia in the brain. Our study provides a novel insight to neurovascular coupling in the brain and will have broad impact in functional brain imaging and diagnosis/ management of brain disease.
Recent Publications:
- Zhou, S., Giannetto, M., DeCourcey, J., Kang, H., Kang, K., Li, Y., Zheng, S., Zhao, H., Simmons, WR., Wei, HS., Bodine, DM., Low, PS., Nedergaard*, M., Wan*, J. (2019) PO2-mediated erythrocyte membrane interactions regulate cerebral capillary hyperemia. Science Advances, 5, eaaw4466.
- Smith, AS., Nowak, RB., Zhou, S., Gokhin, DS., Papoin, J., Ghiran, I., Blanc, L., Wan, J., Fowler*, VM. (2018) Myosin IIA interacts with the spectrin-actin membrane skeleton to control the membrane curvature and deformability of red blood cells. Proc. Nat. Acad. Sci. USA, 115, E4377-85.
- Wei, H., Kang, H., Rasheed, I-Y., Luo, N., Zhou, S., Lou, N., Gershteyn, A., McConnel, E., Wang, Y., Richardson, K., Palmer, A., Xu, C., Wan, J.,* Nedergaard, M.* (2016) Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron, 91, 851-86
Organ-on-a-Chip and 3D Printing
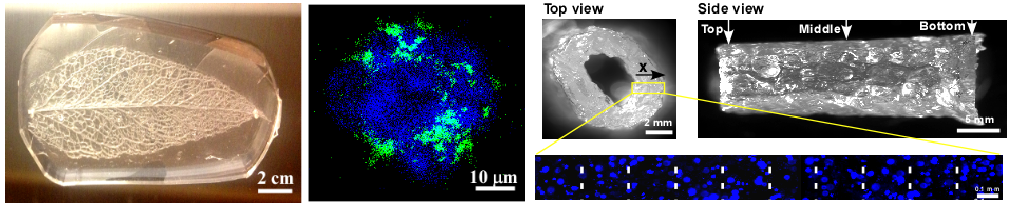
Organ-on-a-chip technology produces 3D mini-organs or tissues in microfluidics, mimicking complex structures and cellular interactions in vivo, and thus provides functional in vitro organ models to study fundamental mechanisms of disease development, drug toxicity screening and drug development. As an emerging concept and a rapid growth field in tissue engineering, organ-on-a-chip technology is expected to revolutionize cell biology in general and the current approaches in tissue engineering and cell culture in particular. My lab is developing microfluidics and 3D bio-printing approaches to construct functional intestine and colon on-a-chip devices by regulating the growth of intestinal organoids in a 3D hydrogel matrix.
Recent Publications:
- Mea, H.J., Delgadillo, L., Wan*, J. (2020) On-demand modulation of 3D-printed elastomers using programmable droplet inclusions. Proc. Nat. Acad. Sci. USA, 117, 14790-14797.
- Piou, M., Fan, R., Darling, E., Cormier, D., Sun, J.,* Wan. J.* (2016) Bioprinting cell-laden Matrigel/agarose constructs. J. Biomater Appl., 0885328216669238.
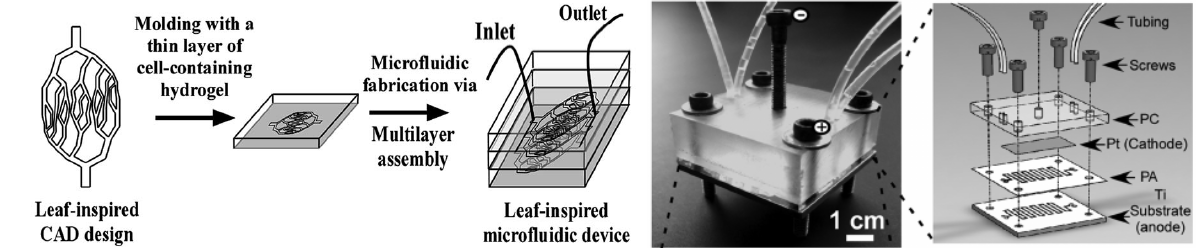
- Fan, R., Sun, Y., Wan, J.* (2015) Leaf-inspired artificial microvascular networks (LIAMN) for 3D cell culture. RSC Advances. 5, 90596-90601.
Microfluidics and Electrochemistry
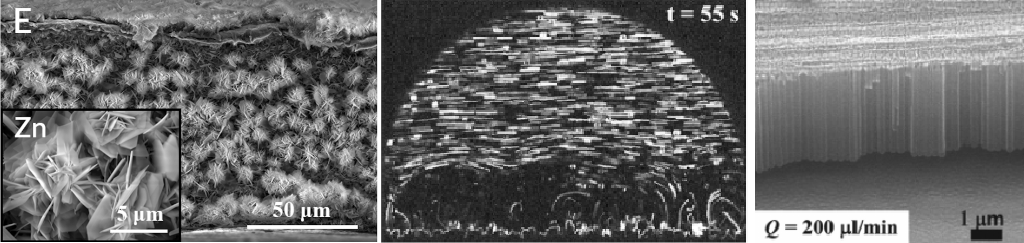
The high surface-area-to-volume ratio, superior heat and mass transfer, controlled reagent mixing and improved reaction rates make microfluidics an attractive approach for multiphase processes, reactions, and material synthesis. My lab investigates the dynamics of emulsion droplets and bubbles in microfluidics and develops approaches to produce functional microbubbles and microparticles. In addition, we investigate electrochemistry in flow conditions and have identified, for the first time, the regulatory roles of flow in the anodic growth of TiO2 nanotubes and demonstrated that flow not only controls the diameter, length, and crystal orientations of TiO2 nanotubes but also regulates the spatial distribution of nanotubes inside microfluidic devices. The demonstrated role of flow in anodic growth of TiO2 nanotubes may apply broadly to a many electrochemical reactions where the local mass transport plays a critical role in reaction kinetics and material synthesis.
Recent Publications:
- Ma, M.C., Li, G., Chen, X., Archer, L.A., Wan, J.* (2021) Suppression of Dendrite Growth by Cross-Flow in Microfluidics. Science Advances. In press.
- Fan, R., Chen, X., Wang, Z., Custer, D., Wan, J.* (2017) Flow-regulated growth of titanium dioxide (TiO2) nanotubes in microfluidics. Small, 13, 1701154. (Featured as a frontispiece article).
- Lu, T., Fan, R., Delgadillo, L., Wan, J.* (2016) Stabilization of carbon dioxide (CO2) bubbles in micrometer-diameter aqueous droplets and the formation of hollow microparticles. Lab On Chip. 16, 1587-159 (Featured as a cover article)
Devices and Technology Development
As much as we are interested in fundamental science, we are passionate to engineering novel devices and explore new technologies to facilitate mechanistic investigations and benefit practical applications. We are currently working on optofluidic devices and ultra-sensitive pressure sensors for neurological research. In addition, we are developing devices for medical diagnostic and battery rejuvenation.
Patents:
- Wan, J., Fan, R., Wang, Z. (2020) Flow-regulated growth of TiO2 nanotubes. US Patent. US20180038006A1.
- Prud'homme, R., Sinko, P. J., Stone, H. A., Pinkerton, N. M.., Shi, L., Wan, J., Ibrahim, S. and Gao, D. (2010) Lung Targeting Dual Drug Delivery System. US Patent. US 9421194B2.